Description
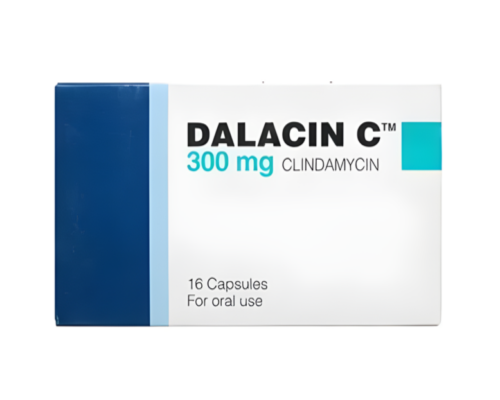
Trade name:
Dalacin C
Composition:
Each capsule contains:
Clindamycin hydrochloride 300 mg
Excipients:
Starch, lactose monohydrate, talc, magnif stearate.
Properties:
Clindamycin is a drug from the group of antibiotics – lincosamides, has a broad spectrum of action, is a bacteriostatic, binds to the 50S subunit of the ribosome and inhibits protein synthesis in microorganisms. Active against Staphylococcus spp. (including Staphylococcus epidermidis, producing penicillinase), Streptococcus spp. (excluding Enterococcus spp.), Streptococcus pneumoniae, anaerobic and microaerophilic gram-positive cocci (including Peptococcus spp. and Peptostreptococcus spp.), Corynebacterium diphtheriae, Clostridium perfringens, Clostridium tetani, Mycoplasma spp., Bacteroides spp. (including Bacteroides fragilis and Bacteroides melaningenicus), anaerobic gram-positive non-spore-forming bacilli (including Propionibacterium spp., Eubacterium spp., Actinomyces spp.).
Indications:
– infectious and inflammatory diseases caused by microorganisms sensitive to clindamycin;
– upper respiratory tract infections and ENT infections (pharyngitis, tonsillitis, sinusitis, otitis), lower respiratory tract infections (pneumonia, including aspiration, lung abscess, pleural empyema, bronchitis), scarlet fever, diphtheria;
– urogenital tract infections (chlamydia, endometritis, vaginal infections, tubo-ovarian inflammation);
– skin and soft tissue infections (infected wounds, abscesses, furuncles, panaritium), abdominal cavity (peritonitis, abscess), oral cavity;
– acute and chronic osteomyelitis;
– septicemia (primarily anaerobic);
– bacterial endocarditis;
– prevention of peritonitis and intra-abdominal abscesses after intestinal perforation or as a result of traumatic infection (in combination with aminoglycosides).
Method of administration and dosage:
Orally, adults and children over 15 years of age (average child weight 50 kg and above) for moderate diseases are prescribed 1 capsule (150 mg) 4 times a day (every 6 hours).
For severe infections, adults and children over 15 years of age, a single dose can be increased to 2-3 capsules (300-450 mg).
Contraindications:
– myasthenia;
– bronchial asthma;
– ulcerative colitis (in anamnesis);
– rare hereditary diseases such as: – galactose intolerance, lactase deficiency or glucose-galactose malabsorption (for capsules);
– pregnancy;
– lactation period;
– children under 3 years of age – for solution for intravenous and intramuscular administration (due to the lack of safety data on the use of benzyl alcohol);
– children under 8 years of age for capsules (the average weight of a child is less than 25 kg);
– hypersensitivity.
Clindamycin is used with caution in patients with severe liver and/or kidney failure, in elderly patients.
Precautions:
Pseudomembranous colitis can occur both during clindamycin administration and 2-3 weeks after stopping treatment (3-15% of cases); it manifests itself as diarrhea, leukocytosis, fever, abdominal pain (sometimes accompanied by the release of blood and mucus with feces).
If these phenomena occur in mild cases, it is sufficient to discontinue treatment and use ion exchange resins (colestyramine, colestipol); in severe cases, compensation for fluid, electrolyte and protein loss, and administration of vancomycin orally or metronidazole are indicated.
Medicines that inhibit intestinal peristalsis should not be used.
Side effects:
From the digestive system: dyspepsia (abdominal pain, nausea, vomiting, diarrhea), esophagitis, jaundice, liver dysfunction, hyperbilirubinemia, dysbacteriosis, pseudomembranous enterocolitis.
From the musculoskeletal system: rarely – impaired neuromuscular conduction.
From the hematopoietic organs: leukopenia, neutropenia, agranulocytosis, thrombocytopenia.
Allergic reactions: rarely – maculopapular rash, urticaria, itching; in some cases exfoliative and vesiculobullous dermatitis, eosinophilia, anaphylactoid reactions.
From the cardiovascular system: with rapid intravenous administration – a decrease in blood pressure, up to collapse; dizziness, weakness.
Local reactions: irritation, soreness (at the site of intramuscular injection), thrombophlebitis (at the site of intravenous injection).
Others: development of superinfection.
Storage method:
Store at a temperature not exceeding 30 degrees.
Packaging:
A cardboard box contains a blister of 10 capsules, paper instructions.